DATA INTEGRITY
What is Data Integrity?
Within “Data Integrity and Compliance with CGMP … Guidance for Industry” document released by the Food and Drug Administration from the United States, the FDA defines Data Integrity as the following …
Data integrity refers to the completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate (ALCOA).
The use of this acronym “ALCOA” is something the FDA uses in other guidance documents as well. Throughout the guidance document, the review of how data is controlled, how audit trails are managed and how records are kept are discussed.
How the Particle Insight complies …
IQ/OQ Qualification Program …
The Pi Sentinel PRO has a robust IQ/OQ Qualification Program which is to be performed by qualified Field Service Engineers (FSE) specially trained and certified by the factory in the execution of the qualification process.
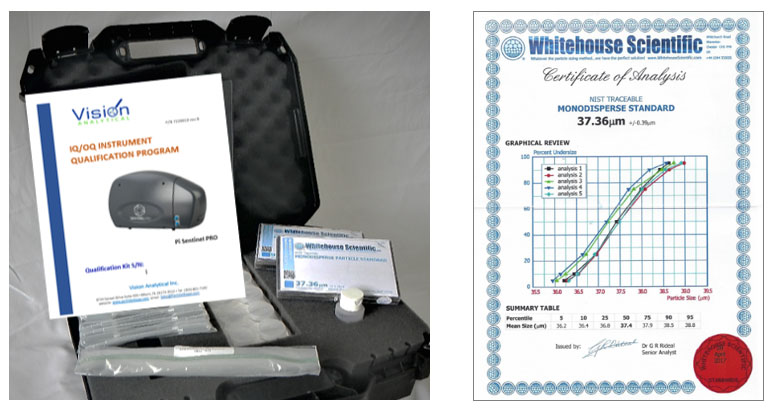
A Qualification Kit provides a set of documents and forms, including a DATA INTEGRITY form, supplies and NIST traceable Standards to complete the Qualification.
Data Integrity form indicates the Pi Sentinel PRO system was set up accordingly to the requirements set forth in Data Integrity section of the IQ/OQ Qualification Program.
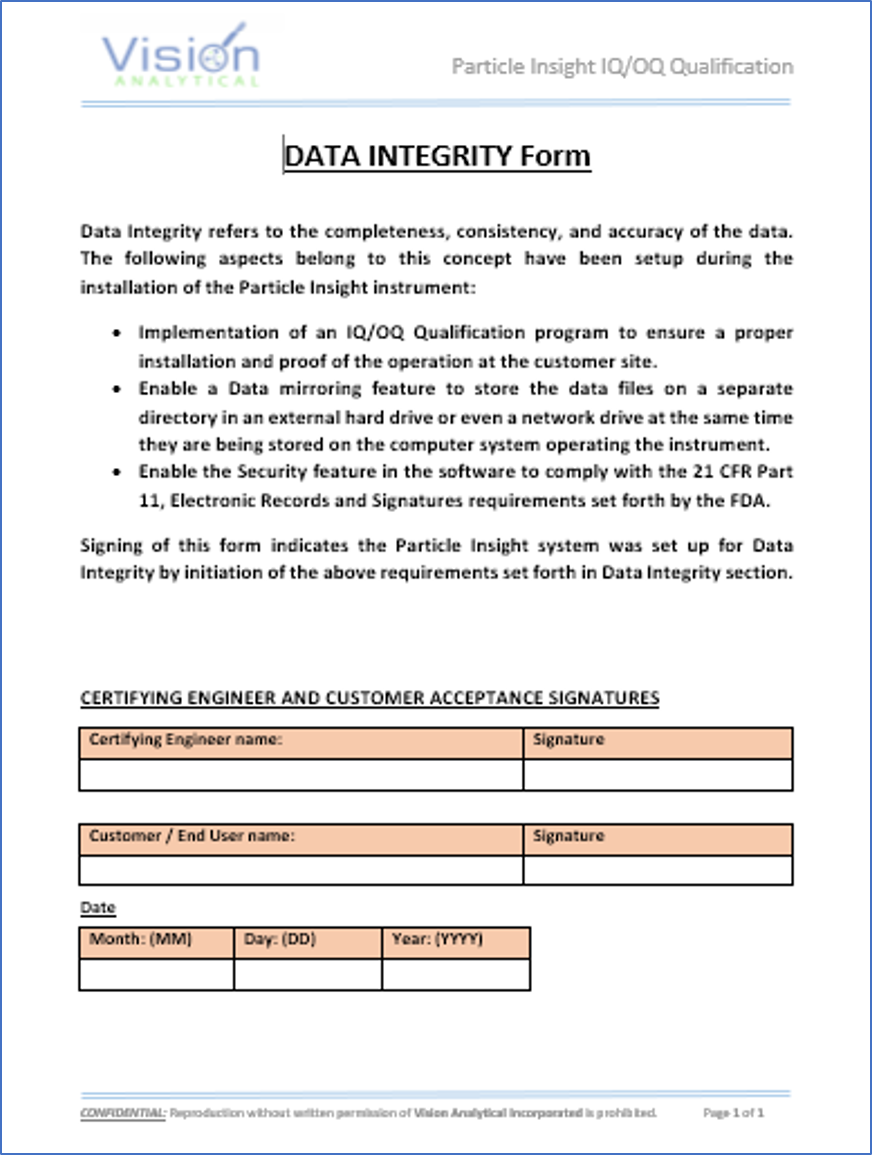
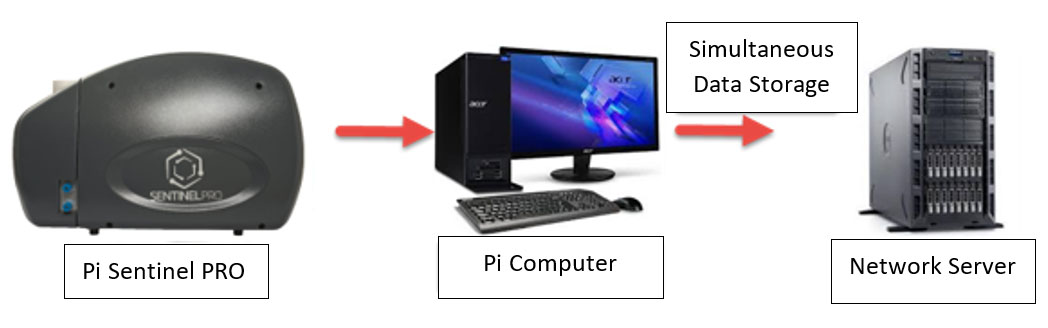
Data Mirroring …
Data Mirroring is a feature within the Pi Sentinel PRO software where the computer software will store the data files on a separate directory or hard drive or even a network drive at the same time it is being stored on the computer system operating the instrument.
For detailed information on how our products are compliant with Data Integrity document below,


